 Researchers at Johns Hopkins are developing a new method called ImmunoMap, a digital map that compares different types of T-cell receptors, the “antennas” found on the surface of T-cells that identify foreign substances in the body to trigger an immune response. ImmunoMap will eventually help physicians to predict which cancer patients will respond best to immunotherapies. A report about the new map appeared in a recent issue of Cancer Immunology Research.
Researchers at Johns Hopkins are developing a new method called ImmunoMap, a digital map that compares different types of T-cell receptors, the “antennas” found on the surface of T-cells that identify foreign substances in the body to trigger an immune response. ImmunoMap will eventually help physicians to predict which cancer patients will respond best to immunotherapies. A report about the new map appeared in a recent issue of Cancer Immunology Research.
How ImmunoMap Will Help Cancer Patients
 According to Jonathan Schneck, MD, PhD, professor of pathology, medicine, and oncology at the Johns Hopkins University School of Medicine, “ImmunoMap gives scientists a picture of the wide diversity of the immune system’s responses to cellular antigens.” Antigens are toxins or foreign substances that stimulate the body’s immune response, particularly with the production of antibodies. The T-cells are responsible for identifying those antigens, and when they do, they send a signal to the immune system to work towards destroying the threat. Although cancer cells do express the antigens that alarm the immune system, they have found ways to escape recognition, which unfortunately allows tumors to keep growing. A lot of cancer immunotherapy treatments are designed to beat this process by eliminating cancer cells, but keeping healthy cells.
According to Jonathan Schneck, MD, PhD, professor of pathology, medicine, and oncology at the Johns Hopkins University School of Medicine, “ImmunoMap gives scientists a picture of the wide diversity of the immune system’s responses to cellular antigens.” Antigens are toxins or foreign substances that stimulate the body’s immune response, particularly with the production of antibodies. The T-cells are responsible for identifying those antigens, and when they do, they send a signal to the immune system to work towards destroying the threat. Although cancer cells do express the antigens that alarm the immune system, they have found ways to escape recognition, which unfortunately allows tumors to keep growing. A lot of cancer immunotherapy treatments are designed to beat this process by eliminating cancer cells, but keeping healthy cells.
As Schneck explained, ImmunoMap will make it easier for physicians to identify which patients are candidates for immunotherapy. Scientists have so far discovered that the more diverse variety of T-cell receptors that a cancer patient has, the better they will take to immunotherapy treatment, and ImmunoMap will help them to learn more about those receptors. “Much of immunotherapy today is built on the premise that we know these antigens, we actually don’t know as much as we need to about them and the T-cells that recognize them,” adds John-William Sidhom, MD and PhD student at Johns Hopkins. In response to this problem, Sidhom developed a mathematical model that functions as a “digital map of the genetic sequence of receptors from human T-cells that were exposed to a virus,” and this model eventually turned into ImmunoMap.
ImmunoMap’s Development
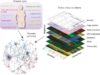
 The researchers hoped to group similar T-cell receptors together, knowing that they might single out the same antigen. The team used high-powered computer technology and an algorithm to map out the arrangement of T-cell receptors and turn the similarities or differences into a matter of distance. Similar receptor sequences would be placed closer together, while different receptor sequences would be placed farther apart. Once the receptors were arranged by distance, the computer could then look for patterns among them. This led to the creation of ImmunoMap, which describes receptor sequences based on their relation to one another, showing that similar T-cell receptors could be identifying the same antigen.
The researchers hoped to group similar T-cell receptors together, knowing that they might single out the same antigen. The team used high-powered computer technology and an algorithm to map out the arrangement of T-cell receptors and turn the similarities or differences into a matter of distance. Similar receptor sequences would be placed closer together, while different receptor sequences would be placed farther apart. Once the receptors were arranged by distance, the computer could then look for patterns among them. This led to the creation of ImmunoMap, which describes receptor sequences based on their relation to one another, showing that similar T-cell receptors could be identifying the same antigen.
The team tested ImmunoMap on tumors in 34 cancer patients who were enrolled in a clinical trial with the drug nivolumab. The three melanoma patients who responded to nivolumab contained more T-cell receptor clusters, about fifteen, whereas the patients who did not respond contained only about eight or nine. The team also discovered that the variety of T-cell receptors decreased by 10-15 percent about a month after the treatment. Schneck believes that “those patients had a broad array of receptor weaponry before their treatment, which may have allowed the right receptor to kill their cancer cells,” and “once their immune system found the correct receptor, T-cells expressing those receptors multiplied, leading to an overall reduction in the structural diversity of their T-cell receptors.” While some scientists believe that immunotherapy response is based upon whether or not T-cells are penetrating the are of the tumor, Schneck’s research proposes that even though this penetration is significant, there are still other factors in play that account for the variation in how patient’s respond to immunotherapy.
ImmunoMap shows great potential in helping to target the right patients for immunotherapy, but it still needs more development before it can correctly predict those outcomes. It can’t yet pair T-cell receptors with antigens or even conclude if those antigens will be a deciding factor in the success of immunotherapy treatments in a given patient. After more research, Schneck hopes that the map will help to create vaccines and “engineered T-cells” to help treat cancer.