The two sides to successful cancer immunotherapy are
- Unique features of the cancer that engender artificially driving cancer-specific immunity (cytotoxic CD8 T cells for example) and/or offer paths exploitable by immunotherapy (antibodies against checkpoint inhibitors such as CTLA-4 or PD-1 for example).
- Potential for effective cancer-specific immune responses.
Cancer Features That Favor Immunotherapy Success
Research shows certain cancer features are conducive to helping initiate and/or sustain effective anti-cancer immunity.
These include
- High level of genomic instability and mutational load. Data supporting this feature observed in immunotherapy success cases against non-small cell lung cancer, melanoma, metastatic melanoma and colorectal cancer.
- To ensure the immune system doesn’t attack the body itself, most tissue antigen-specific T (and B) cells never make it through developmental bottlenecks. In other words, normal immunological tolerance processes that safeguard body from attack by its own immune system are also a natural barrier to strong anti-tumor immunity.
- What is a cancer cell? A cell that some time in the past went rogue, no longer subject to growth control. What does that signify to the immune system though? Does it remain a cell the immune system’s been programmed to tolerate or something different that it can recognize and respond to? Having started out as a normal tissue cell, most of what a tumor cell expresses is the same as that tissue.
- The crux that determines whether or not immunotherapy can even be harnessed to rid of a tumor is how antigenically different it’s become from a normal cell.
- More antigenically similar a tumor to normal tissue, lower the chances of tumor-specific immune cells. After all, to even be able to make effective anti-tumor immune responses in the first place, one needs tumor-specific T cell (CD4 and CD8).
- Since the human body’s immune system is geared to push through T and B cells specific for antigens not expressed by it in the normal course of a lifetime, neoantigen-specific T and B cells are to be expected.
- Thus, more neoantigens a tumor expresses, greater the likelihood they can be ‘seen’ by the immune system, greater the likelihood of higher frequencyof neoantigen-specific cytotoxic CD8 T cells, and greater the chances of being able to artificially stoke effective tumor-specific immunity.
- Presence of Tumor-infiltrating lymphocytes (TIL). More TILs, especially cytotoxic CD8s, more immunogenic the tumor and tumor-associated cells, i.e., capable of eliciting anti-tumor-specific immunity. Most cancer immunotherapies try to maximize targeted killing by cytotoxic CD8 T cells, which is considered the main, most effective anti-tumor immune cell type and response. Such approaches work poorly in non-immune cell solid tumor patients who have few such cells to start with, for e.g., prostate cancer.
- OTOH, tumors with few or no TILs could mean (see below from 10)
- Tumor actively restricts their entry. For e.g., by releasing specific chemokines such as nitrated CCL2.
- Tumor vasculature expresses specific molecules such as Fas ligand capable of causing T cell apoptosis (death) or Prostaglandin E2 capable of blocking their effector response.
- Tumor may maintain low oxygen levels in and around it, Hypoxia (medical). In turn, such conditions promote Programmed cell death protein 1 expression on tumor-associated antigen-presenting cells. PD-1 binding to PD-L1 on CD8 T cells inhibits their response.
- Tumor microenvironment may favor accumulation of metabolites such asIndoleamine 2,3-dioxygenase (IDO) which inhibit CD8 T cell response.
- Tumor may specifically recruit certain fibroblasts and B cells to its vicinity toinhibit CD8 T cell entry and responses, respectively.
- In other words, successful cancer immunotherapy against solid tumors has to be carefully engineered in order to prevail over numerous natural physical and functional obstacles.
Features of effective anti-cancer immunity
- More TILs and more CD8s among such TILs.
- High level of PD-L1 expression. Even though high PD-L1 expression in tumors inhibits effective anti-tumor immunity by binding to PD-1 on CD8 T cells and inhibiting them, targeting PD-1 or PD-L1 through specific mAbs (Monoclonal antibody) is akin to lifting the brakes from these cells, unleashing effective anti-tumor immunity.
- Interferon gamma is an important cytokine in the cytotoxic T cell’s armamentarium. A 2016 study finds melanomas resistant to Ipilimumab, mAb against checkpoint inhibitor CTLA-4, lose IFN-gamma signaling capacity, i.e., plausible reason for their resistance.
Assays And Biomarkers Used To Measure Anti-Tumor Immunity During Immunotherapy
Cellular Techniques To Assess Immune Status Within Tumors
- IHC (immunohistochemistry) is an old workhorse that’s used to count the number of TILs in a tumor tissue using anti-CD8 antibodies. Now anti-PD-L1 antibodies are also being used to assess PD-L1 expression as a biomarker for how useful anti-PD-1 or -PD-L1 antibodies might be in releasing the brakes to unleash anti-tumor immune responses from TILs present within it. Such assessments have caveats because different studies showed different predictive power of anti-PD-L1 IHC.
- Different studies used different antibodies and used different thresholds for assessing positivity.
- An inducible cell-surface molecule, PD-L1’s presence or absence in archival tissue samples can’t fully predict its real-time status.
- Immunoscore, a relatively new pathology algorithm developed by French pathologist, Jérôme Galon, uses digital pathology to minimize variability and provides quantitative data on T cells within a tumor, not just in its center but also in its margins, something that improves prognostic accuracy. This approach is slowly working its way through to widespread validation and maybe eventual acceptance.
- Currently hobbled by technical limitations and interpretation difficulties, multiplex IHC, i.e., trying to assess multiple tissue- and cell-specific markers simultaneously, would provide more information not only on numbers of immune cells within tumors but also their spatial organization. Sequential multiplex IHC is an approach to make this technology workable, but it’s still far from practical utility.
- Cheap, long-lasting, FFPE (formalin fixed paraffin embedded) tissue sections are a mainstay in pathological diagnoses. Mass spectrometry based FFPE multiplexing is an attempt to marry this ancient technique with a more modern molecular analytical tool to exponentially increase the number of markers assessed to as many as 100. A 2014 study used this approach to simultaneously image as many as 32 breast cancer-associated hormonal and immunological proteins.
- Flow cytometry & Mass cytometry (CyTOF). Of more value in blood cancers rather than solid tumors, using antibodies labeled with rare metals, CyTOF (cytometry in time-of-flight) combines flow cytomtery and mass spectromtery. A 2015 study simultaneously assessed 15 surface and 16 intracellular proteins in leukemia and showed mismatch between surface and intracellular states of some of these proteins while also being able to identify that cell cycle differences between leukemia stem cells could influence their response to therapy.
Genomic Techniques To Assess Immune Status Within Tumors
- Exome sequencing (Whole Exome Sequencing, WES) of tumor tissue allows its mutational load estimation. Combining this with algorithms that help predict likelihood that peptide sequences bind to HLA (Human leukocyte antigen) or help predict likelihood TCRs (T cell receptors) will recognize them could improve Rx outcomes in a more individualized manner.
- DNA mismatch repair (MMR) deficiency could be a biomarker of response to PD-1 based immunotherapy, especially in colorectal cancers.
- T cell receptor (TCR) sequencing can monitor changes in T cell populations within a tumor over the course of Rx. Differences between responders and non-responders might identify those more likely to benefit from Rx.
- Describing a tumor’s transcriptional activity, RNA sequencing is to RNA what WES is to DNA.
- While all these approaches have been attempted on tumor tissues, newer approaches seek to apply them at the single cell level. One such approach showed for example that mutational patterns in each cancer cell in acute myeloid leukemia patients wasdifferent.
- Results such as these reveal why even the latest immunotherapies end up benefiting only a handful of patients and why realistic cancer cure may only come from individualized, and hence extremely costly, efforts.
- Most of these approaches are invasive, requiring access to tumor itself. A non-invasive counterpart tries to take advantage of tumor metabolite spillover into bloodstream.
- Exosome (vesicle) are 50 to 100 nm membrane-bound vesicles secreted by many cells, including tumor and immune cells. They’re thought to be a way for cells to communicate over short and long distances by exchanging genetic and protein material. An as-yet unpublished 2015 study that used analysis of circulating exosomes to assess responses to cancer immunotherapy founddifferences between responders and non-responders (30), suggesting such differences could be used as predictive biomarkers, if found reproducible.
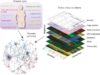
See composite pictorials below on cellular and genomic approaches currently used or proposed to be used to monitor anti-tumor immunity during immunotherapy in some clinical trials.
Practical Obstacles to Studying Human Anti-Tumor Immunity
- Kind of tumor tissue available for assessment makes a huge difference, specifically whether tissue is archival or fresh (see below from. Advantages of the former are to the patient, making another invasive biopsy unnecessary. OTOH, disadvantages include changes to cell, protein and gene expression profiles depending on how archival tissue’s been preserved. Obviously, fresh tumor tissue reflects current disease state more accurately, particularly changes in response to Rx.
- Since tumor and immune cell features are dynamic, not static, sampling over time may yield more accurate information. Obviously this increases cost and may also increase risk to patient.
- PD-L1 represents an excellent example of pitfalls inherent to choice of tumor tissue and sampling frequency used in cancer immunotherapy decision making. On the one hand, it’s now well appreciated that higher the PD-L1 expression in tumors, better the chances of Cancer immunotherapy. However, most of the time, PD-L1 expression is assessed on archival tissue. Since PD-L1 is an inducible molecule, making Rx decisions on results from archival tissue thus carries a two-fold burden, one, passage of time since that sample was taken, and two, dynamic changes in PD-L1 expression in tumor.
- Amount of tissue available is another important variable.
- Core biopsies are too small to enable meaningful immunological assessments such as topographic TIL enumeration, i.e., how many are present in the tumor center versus its invasive margins.
- Tumors can also be highly heterogeneous, not just at different sites but also within the same tumor.
- More sampling at more places gives a more accurate picture but again at higher cost and risk to the patient.