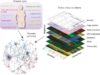
San Diego, CA – Novel Cancer Vaccine ValloVax from Batu Biologics Aims to Starve Tumors by Targeting Multiple Cell-Signaling Pathways
 Cancer vaccines may be more effective against tumorgenesis if they target multiple rather than single angiogenesis signaling pathways, contend scientists with Batu Biologics, a San Diego, California, biotechnologyfirm currently developing a promising immunotherapy called ValloVax that deprives non- small cell lung cancer of its ability to locally override the immune system and to form cellular vasculature.
Cancer vaccines may be more effective against tumorgenesis if they target multiple rather than single angiogenesis signaling pathways, contend scientists with Batu Biologics, a San Diego, California, biotechnologyfirm currently developing a promising immunotherapy called ValloVax that deprives non- small cell lung cancer of its ability to locally override the immune system and to form cellular vasculature.
Batu Biologics researchers assert that most U.S. Food and Drug Administration-approved anti- angiogenesis therapeutics are designed to disrupt or inhibit activity along a single cell-growth pathway. This, they explain, is why those cancer-fighting strategies are proving to be less successful than hoped atslowing or stopping tumor progress.
The researchers say the multiple pathway approach is superior because it trains the immune system to not merely detect a tumor trying to conceal itself from lymphocyte attack but, pivotally, to also strike at the malignancy through its “Achilles heel” – the vasculature each cancer cell constructs to permit intake of needed growth- and metastatic-supporting oxygen and nutrients.
Multiple Pathways Regulate Angiogenesis
Angiogenesis is the process by which cancer cells develop blood vessels.1 These blood vessels are essential to the survival, growth, and spread of tumors.2 New blood vessel formation (angiogenesis) is fundamental to tumor growth, invasion, and metastatic dissemination.
Angiogenesis is regulated by a number of signaling pathways, most prominently vascular endothelial growth factor (VEGF).3 Many studies have demonstrated the value of targeting VEGF in order to inhibit angiogenesis,4 and, indeed, most of the anti-angiogenesis therapeutics currently approved by the FDA for indications of cancer are designed to inhibit this one signaling pathway to the exclusion of all others.
Another reason to inhibit angiogenesis is that doing so reduces the ability of cancer cells to shield themselves from immune system responses. When the immune system detects the presence of cancerous cells, one way it responds is by swarming them with locally produced cytotoxic T-lymphocytes (CTLs).5
These CTLs have the capacity to bind to cancer cells, penetrate them, and, ultimately, kill them.6 However, angiogenesis enables cancer cells to inactivate CTLs as they bind to the ligands expressed along the tumor endothelium.7 Most consequential among these ligands is PD-L1 (programmed death lig and 1).8
Moreover, tumors of sufficient size secrete cytokines that prevent CTLs from even recognizing cancer cells in the first place. This causes CTLs to either mute their attacks or simply not attack at all.
ValloVax Disrupts Along Multiple Pathways
Conventionally, cancer drugs attempt to block angiogenesis by employing one of two approaches. The first entails use of antibodies. The second involves delivery of small-molecule tyrosine kinase inhibitors.
Bevacizumab (tradename Avastin; developer Genentech) is an example of an antibody-based angiogenesis inhibitor that targets VEGF. Sunitinib malate (tradename Sutent; developer Pfizer) is an example of a small-molecule tyrosine kinase-based, VEGF-targeting angiogenesis inhibitor.
Batu Biologics researchers indicate that the critical deficiency of these approaches is they target VEGF and no other signaling pathway. “The two approaches do not take into account that cancer angiogenesis is driven by a variety of tumor secreted factors,” the scientists say.
Therefore, blocking only VEGF, while useful, still permits cancer cells to flourish, the researchers indicate.
“We believe that a better approach for blocking tumor angiogenesis is to target multiple pathways concurrently,” the researchers write. “ValloVax is engineered to train the immune system to disrupt angiogenesis and in so doing interfere with development of cancer-cell vasculature, thereby curtailing blood supply to the tumor.”
The researchers say this multiple pathways targeting approach creates the potential for achieving superior efficacy – in part possible because ValloVax may help the immune system attain improved access to its cancerous targets.
“The immune system is in direct contact with the tumor vasculature via the blood,” the researchers explain. “Often, tumor-associated protein markers, or antigens, are very hard to reach and are sequestered from the patient’s immune system. In early testing, we see evidence that ValloVax may be able to reduce or perhaps even eliminate this problem.”
ValloVax in FDA Approval Pipeline
Batu Biologics investigators note that ValloVax augments immune system penetration of tumors. “ValloVax is designed to ‘punch holes’ in the tumor endothelium allowing CTLs to gain better access to the cancer cells’ internal machinery,” they explain.
“An important reason we are targeting the tumor endothelium with ValloVax is to prevent the vasculature from establishing resistance to other cancer drugs,” they add.
“Another important reason is to prevent tumors from attaining sizes greater than 2mm in diameter.”
Batu Biologics has licensed the manufacture of ValloVax to the University of Miami.
The company’s pre-clinical work on the cancer vaccine is completed and expects the FDA will grant it Investigational New Drug (IND) status later this year.
IND designation will clear the way for ValloVax to begin a Phase I clinical trial of ValloVax on humans diagnosed with non-small- cell lung cancer. That form of cancer is only one of several for which ValloVax eventually might be indicated (already Bantu Biologics has identified with the aid of mouse models colon cancer, breast cancer, melanoma, and glioblastoma as malignancies potentiallytreatable using
ValloVax).
REFERENCES
1-4 Niu G, Chen X. Vascular Endothelial Growth Factor as an Anti-angiogenic Target for Cancer Therapy.
Current drug targets. 2010;11(8):1000-1017.
5-8 Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting
programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580-6587.